Beta Amyloid Antibodies and Antigens for Alzheimer's Research
The beta amyloid antibody targets amyloid plaques, a key Alzheimer's disease hallmark. Engineered to
recognize and neutralize amyloid proteins, these antibodies potentially slow the disease's progression by
preventing plaque formation in the brain, representing a significant stride in Alzheimer's research and
therapeutic approaches, aiming to improve patient care and outcomes.
| Catalog No. | Products Name | Biomarker | Fc/Tag | Products Information |
| GMP-h-Aβ42-Ab | Anti-human Aβ42 monoclonal antibody (mAb) | beta-amyloid 42 (Aβ42) | hFc/mFc | Details |
| GMP-h-Aβ40-Ab | Anti-human Aβ40 monoclonal antibody (mAb) | beta-amyloid 40 (Aβ40) | hFc/mFc | Details |
| GMP-h-Aβ42-Ag01 | Beta-amyloid 42 (Aβ42) antigen | beta-amyloid 42 (Aβ42) | Details | |
| GMP-h-Aβ40-Ag01 | Beta-amyloid 40 (Aβ40) antigen | beta-amyloid 40 (Aβ40) | Details |
Validation of GeneMedi's Aβ40/Aβ42 products
The high-performance Aβ40 and Aβ42 antibody raw materials developed by GeneMedi have the advantages of good specificity and high sensitivity, and are the preferred raw materials for the development of in vitro diagnostic reagents for Alzheimer's disease.
Good performance of GeneMedi's Aβ40/Aβ42 antibodies in ELISA and CLIA Validation
The high-performance Aβ40 and Aβ42 antibody raw materials developed by GeneMedi have the advantages of good specificity and high sensitivity, and are the preferred raw materials for the development of in vitro diagnostics reagents for Alzheimer's disease.
In direct ELISA analysis, GMP-h-Aβ40-Ab01 and GMP-h-Aβ42-Ab01 can Recognizes Aβ40 or Aβ42 with high specificity. GMP-h-Aβ42-Ab02 has cross-reactivity with both Aβ40 and Aβ42.
In CLIA verification, the antibody pair (Capture: GMP-h-Aβ40-Ab01 , Detect: GMP-h-Aβ42-Ab02 ) can detect the GMP-h-Aβ40-Ag01, and the antibody pair (Capture: GMP-h-Aβ42-Ab02 , Detect: GMP-h-Aβ42-Ab01 ) can detect the GMP-h-Aβ42-Ag01. Beta-amyloid(1-42) antibodies (GMP-h-Aβ42-Ab02 and GMP-h-Aβ42-Ab01) established a standard curve, and the detection range of Aβ42 was 100-1700 pg/mL.
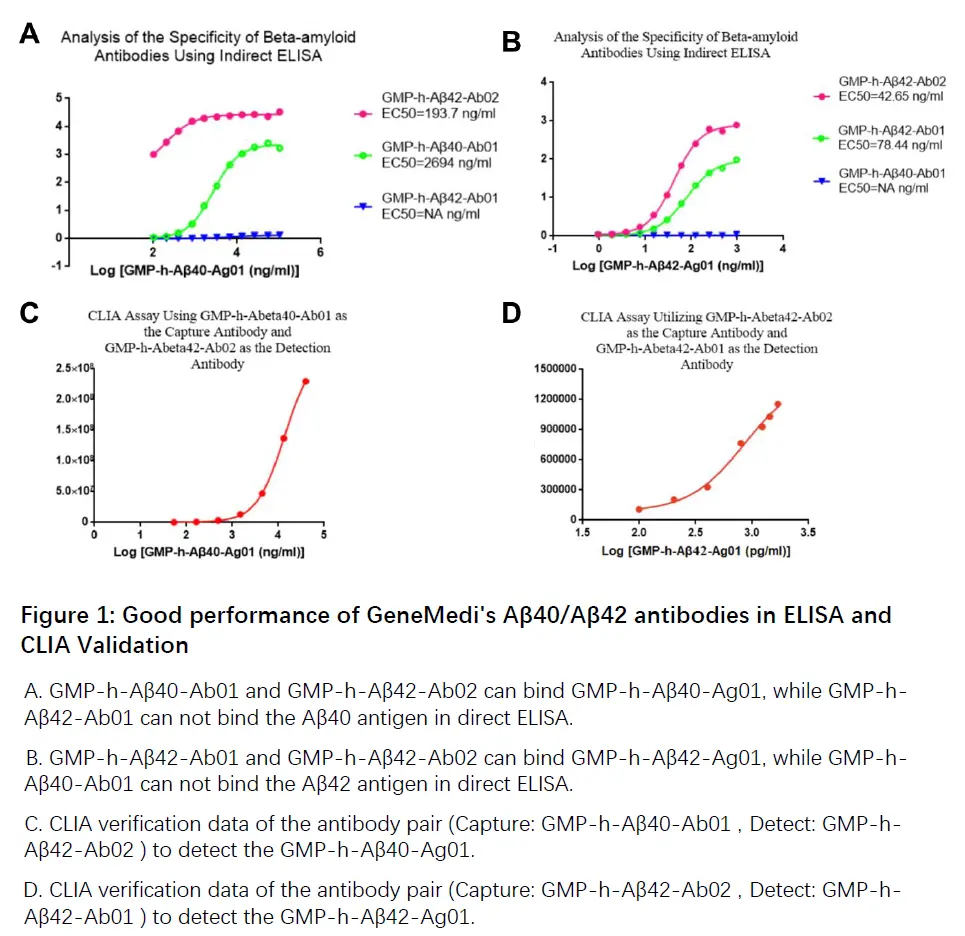
Figure 1: Good performance of GeneMedi's Aβ40/Aβ42 antibodies in ELISA and CLIA Validation
A. GMP-h-Aβ40-Ab01 and GMP-h-Aβ42-Ab02 can bind GMP-h-Aβ40-Ag01, while GMP-h-Aβ42-Ab01 can not bind the Aβ40 antigen in direct ELISA.
B. GMP-h-Aβ42-Ab01 and GMP-h-Aβ42-Ab02 can bind GMP-h-Aβ42-Ag01, while GMP-h-Aβ40-Ab01 can not bind the Aβ42 antigen in direct ELISA.
C. CLIA verification data of the antibody pair (Capture: GMP-h-Aβ40-Ab01 , Detect: GMP-h-Aβ42-Ab02 ) to detect the GMP-h-Aβ40-Ag01.
D. CLIA verification data of the antibody pair (Capture: GMP-h-Aβ42-Ab02 , Detect: GMP-h-Aβ42-Ab01 ) to detect the GMP-h-Aβ42-Ag01.
Understanding Beta Amyloid
The 2023 Alzheimer's Association International Conference (AAIC) released a draft proposal for AD diagnostic standards, which introduces blood-based biomarker testing for the first time. AD can be diagnosed through plasma markers such as Aβ42, Aβ40, P-tau181, P-tau217, P-tau231, NFL, GFAP (as shown in the table above). These markers have not yet been subjected to large-scale clinical studies, and relevant groups need to be Do further analysis. For the diagnosis of AD, it is necessary to rely on comprehensive methods such as clinical manifestations and imaging.
Aβ40 polypeptide and Aβ42 polypeptide are common forms found in human cerebrospinal fluid and serum. It is generally believed that Aβ42 peptide has stronger neurotoxicity and is more prone to aggregation. Changes in Aβ42 peptide concentration in human cerebrospinal fluid and blood are associated with Alzheimer's disease.
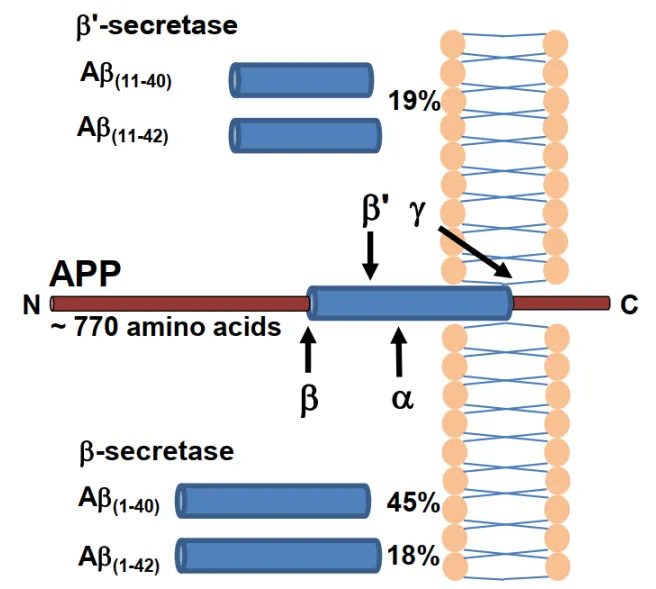
Beta Amyloid is produced by the hydrolysis of amyloid precursor protein (APP) by β and γ secretases and is composed of 38-43 amino acids with toxic effects (as shown on the right ).
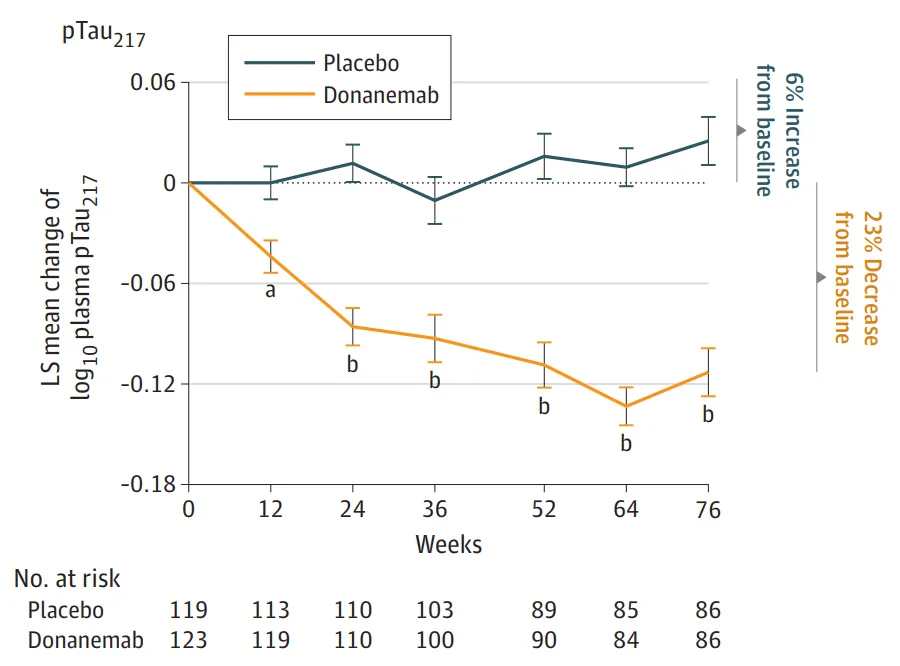
Eli Lilly's new drug Donanemab is an investigational drug for the treatment of AD. It is a specific antibody targeting the N-terminal pyroglutamate Aβ epitope of amyloid plaques. Donanemab antibody can significantly reduce blood pTau217 levels. A significant decrease in plasma pTau217 levels was observed after 12 weeks of treatment with donanemab compared with placebo and was sustained throughout the 76 weeks of the study.
Mean plasma pTau217 levels in the donanemab treatment group decreased by 23% from baseline. In contrast, mean plasma pTau217 levels in the placebo group continued to increase by 6% from baseline to the end of the study, as shown below. pTau217 may become a companion diagnostic indicator in the treatment of AD by Donanemab.






