Diagnostic antibodies and antigens for functional ELISA assay kit, lateral flow and other immunoassays in diagnostics
GeneMedi's product list for ln-vitro Diagnostics (IVD)
Category
About In vitro diagnostic (IVD)
In vitro diagnostic (IVD) testing has become an indispensable tool in clinical
practice for
diagnosing and monitoring of diseases, as well as providing prognosis and
predicting treatment
response, In vitro tests may be done in laboratories, health care facilities or
even in the home.
The tests themselves can be performed on a variety of instruments ranging from
small, handheld tests
to complex laboratory instruments. They allow doctors to diagnose patients
effectively and work to
provide appropriate treatments.
IVDs are used to analyze human samples such as blood and saliva, either by
measuring the
concentration of specific substances, or analytes (such as sodium and
cholesterol), or by detecting
the presence or absence of a particular marker or set of markers, such as a
genetic mutation or an
immune response to infection. Clinicians regularly use IVDs to diagnose
conditions, guide treatment
decisions, and even mitigate or prevent future disease (for example, through
screening tests that
indicate a patient’s risk of developing a given condition in the future). There
are over 40,000
different IVD products available that provide information to doctors and
patients on a huge range of
conditions. These comprise markers for inorganic chemistry (electrolytes,
toxins, and heavy metals),
markers for organic chemistry/biochemistry (proteins, lipids, and
carbohydrates), as well as
molecular biologic procedures (sequencing and polymerase chain reaction).
In recent years, the value of IVDs has gained further recognition because of
their potential to save
lives through earlier diagnosis of medical conditions. In the case of an
infectious disease, early
detection can ensure that patients receive timely care to help slow or stop the
progression of
disease. Further, knowledge of infection can help promote self-isolation to
prevent further spread
of the disease to the community. This has been particularly important in
containing the spread of
highly infectious agents during an active outbreak. Using IVDs to predict
underlying genetic
conditions in addition to diagnostic screening can also prevent unnecessary
suffering by a patient
as well as reduce the scale of treatment required.
As the ease-of-use and reliability of IVDs has improved, some IVDs have moved
out of the laboratory
setting and into the patient’s home. Consumers can now perform simple tests to
monitor blood glucose
levels, identify urinary tract infections, track ovulation, and confirm
pregnancy. These tests not
only empower individuals to take charge of their own health but can provide
quick results that help
guide further medical actions. Genemedi provides diagnostic antibodies and
antigens for the in vitro
diagnosis of diseases such as infectious disease,
inflammation/autoimmune/inflammatory disease,
kidney function (renal damages), liver diseases, lung injury, metabolic
diseases, multiple disease,
neurodegenerative diseases, pain, thyroid diseases, vitamin deficiency and so
on.
Validation Data
Evidence has shown plasma biomarkers (p-tau181, p-tau217, p-tau231, p-tau202 and
205, p-tau212 and
214, p-tau413, p-tau422, GFAP, NfL, and Aβ42/40) were significantly changed in
preclinical
Alzheimer's Disease (AD). Recently, our R&D department demonstrated that our
GMP-h-p-tau217-Ab01 has
a large linear range and good sensitivity against the GMP-h-p-tau217-Ag01. Below
is the result of
GeneMedi's GMP-h-p-tau217-Ab01 (Anti-human p-tau217 antibody) validation with
GMP-h-p-tau217-Ag01
(p-tau217 antigen) in ELISA. We highly recommend the Ab&Ag to you.
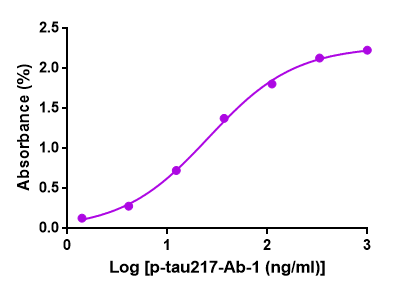 Figure 1. GeneMedi's GMP-h-p-tau217-Ab01 (Anti-human p-tau217 antibody) is
validated to detect
the GMP-h-p-tau217-Ag01 (p-tau217 antigen) in ELISA. EC50 =
25.85ng/ml.
Figure 1. GeneMedi's GMP-h-p-tau217-Ab01 (Anti-human p-tau217 antibody) is
validated to detect
the GMP-h-p-tau217-Ag01 (p-tau217 antigen) in ELISA. EC50 =
25.85ng/ml.






 Facebook
Facebook LinkedIn
LinkedIn Twitter
Twitter
