Anti-payload antibody in PK study in ADC drug development
Anti-payload antibodies for ADCs would add another layer of safety and efficacy by further minimizing effect from free cytotoxic agents. They assist with targeting specific drugs, enhancing the therapeutic index and helping monitor drugs, a definite step forward in targeted cancer treatment with enhanced prospects of individualized therapies.
| Cat No. | Payload | Product Name | Fc | Technical Information | Products Information |
| GTU-Bios-Maytansinoids-Ab | DM1/DM4 | Anti-DM1/DM4 monoclonal antibody(mAb) | hFc/mFc | More | Details |
| GTU-Bios-Auristatin-Ab-01 | MMAE/MMAF | Anti-MMAE/MMAF monoclonal antibody(mAb) | hFc/mFc | More | Details |
| GTU-Bios-Auristatin-Ab-02 | MMAE (Specific) | Anti-MMAE (Specific) monoclonal antibody(mAb) | hFc/mFc | More | Details |
| GTU-Bios-DXd-Ab | DXd/Exatecan | Anti-DXd&Exatecan monoclonal antibody(mAb) | hFc/mFc | More | Details |
| GTU-Bios-CPT-Ab | Camptothecin (CPT) | Anti-CPTmonoclonal antibody(mAb) | hFc/mFc | More | Details |
| GTU-Bios-Eribulin-Ab | Eribulin | Anti-Eribulin monoclonal antibody(mAb) | hFc/mFc | More | Details |
| GTU-Bios-Exatecan-Ab | Exatecan | Anti-Exatecan monoclonal antibody(mAb) | hFc/mFc | More | Details |
| GTU-Bios-SN-38-Ab | SN-38 | Anti-SN-38 monoclonal antibody(mAb) | hFc/mFc | More | Details |
| GTU-Bios-Budesonide-Ab | Budesonide | Anti-Budesonide monoclonal antibody (mAb) | hFc/mFc | More | Details |
| GTU-Bios-MTX-Ab | MTX | Anti-MTX monoclonal antibody(mAb) | hFc/mFc | More | Details |
| GTU-Bios-PBD-Ab | PBD | Anti-PBD monoclonal antibody(mAb) | hFc/mFc | More | Details |
| GTU-Bios-PNU-159682-Ab | PNU-159682 | Anti-PNU-159682 monoclonal antibody(mAb) | hFc/mFc | More | Details |
| GTU-Bios-Amanitin-Ab | Amanitin | Anti-Amanitin monoclonal antibody(mAb) | hFc/mFc | More | Details |
| GTU-Bios-Calicheamicin-Ab | Calicheamicin | Anti-Calicheamicin monoclonal antibody(mAb) | hFc/mFc | More | Details |
| GTU-Bios-Doxorubicin-Ab | Doxorubicin | Anti-Doxorubicin monoclonal antibody (mAb) | hFc/mFc | More | Details |
| GTU-Bios-Duocarmycin-Ab | Duocarmycin | Anti-Duocarmycin monoclonal antibody (mAb) | hFc/mFc | More | Details |
GeneMedi's Case study of Anti-payload antibody
- MMAE/MMAF
- DXd
- Exatecan

Figure 1. GTU-Bios-Auristatin-Ab-01-2 and GTU-Bios-Auristatin-Ab-02-2 have been actively bound with the ADC (PTM-1 MMAE).
Figure 1 demonstrates the efficacy of
GeneMedi's GTU-Bios-Auristatin-Ab-01-2
and GTU-Bios-Auristatin-Ab-02-2 in
actively binding with the ADC (PTM-1 MMAE). This underscores GeneMedi's
commitment to
producing
high-quality antibodies tailored for effective conjugation with MMAE-based ADCs.
The robust
binding
displayed in the figure highlights GeneMedi's expertise in developing antibodies
optimized
for
targeted drug delivery, a critical aspect in the field of antibody-drug
conjugates.

Figure 2. The GTU-Bios-Auristatin-Ab-01-2 has been actively bound with the ADC (PTM-1 MMAF). While GTU-Bios-Auristatin-Ab-02-2 has not been bound with the ADC with MMAF.
In Figure 2, the specificity of
GeneMedi's antibodies is showcased
as GTU-Bios-Auristatin-Ab-01-2 successfully
binds with the ADC (PTM-1 MMAF), while GTU-Bios-Auristatin-Ab-02-2 remains unbound. This
specificity
is
pivotal in ensuring precise targeting of MMAE-containing ADCs, enhancing their
therapeutic
efficacy
while minimizing off-target effects.
GeneMedi's ability to tailor antibodies to selectively
bind
with distinct drug payloads exemplifies their proficiency in antibody
engineering, offering
researchers reliable tools for precision medicine applications.
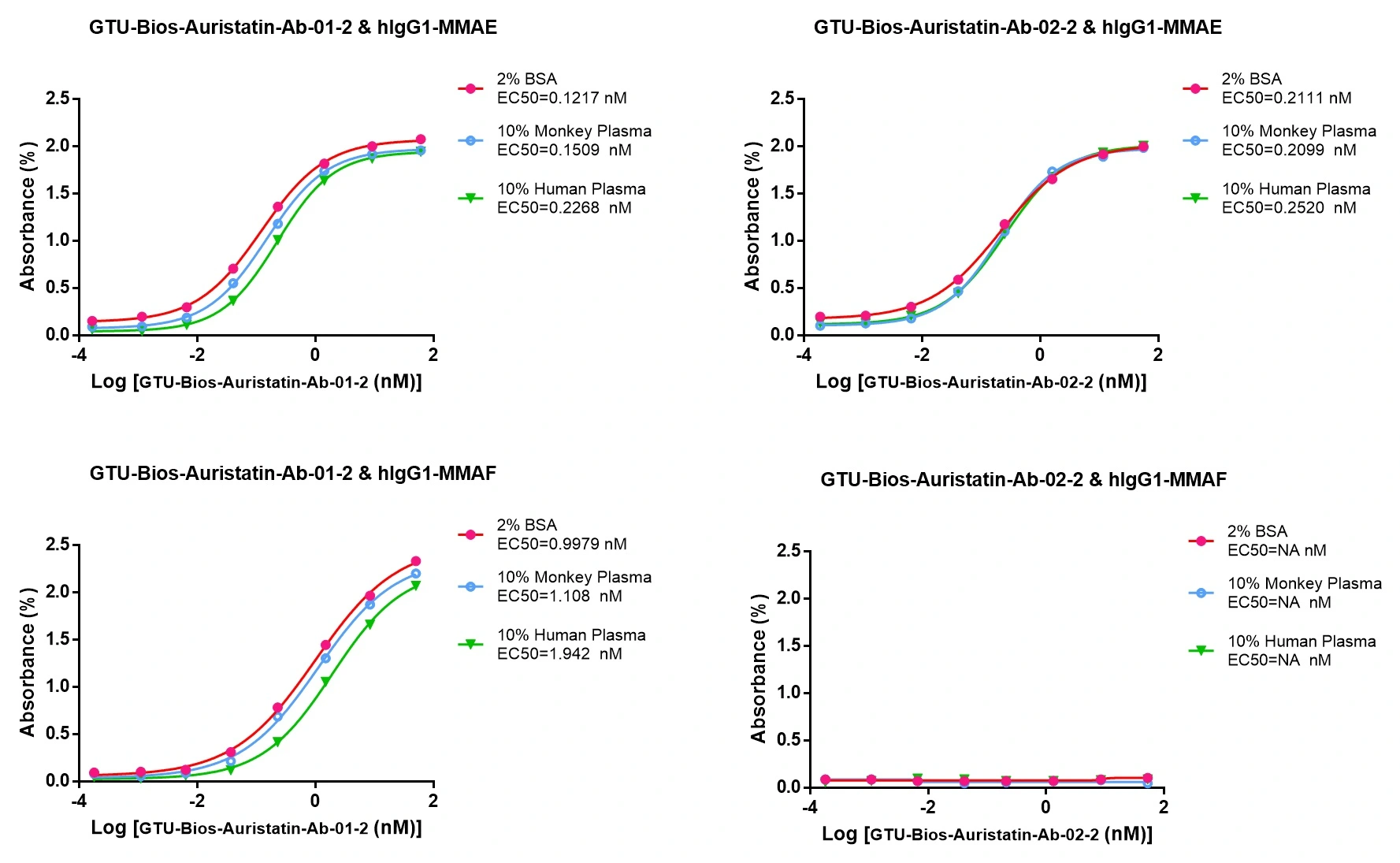
Figure 3. GTU-Bios-Auristatin-Ab-01-2 and GTU-Bios-Auristatin-Ab-02-2 can be used in pharmacokinetic (PK) studies involving humans and monkeys
GTU-Bios-Auristatin-Ab-01-2 has minimal impact in human and monkey PK studies and can be considered negligible. GTU-Bios-Auristatin-Ab-02-2 has even less impact than GTU-Bios-Auristatin-Ab-01-2 in these PK studies. Both GTU-Bios-Auristatin-Ab-01-2 and GTU-Bios-Auristatin-Ab-02-2 are suitable for use in human and monkey PK experiments.

Figure 4. GTU-Bios-Auristatin-Ab-01-2 and GTU-Bios-Auristatin-Ab-02-2 have not been bound with the ADC conjugated with DXD.
Figure 4 illustrates another aspect of GeneMedi's antibody specificity, revealing that neither GTU-Bios-Auristatin-Ab-01-2 nor GTU-Bios-Auristatin-Ab-02-2 binds with the ADC conjugated with DXD. This data underscores GeneMedi's dedication to producing antibodies with high selectivity, ensuring minimal interference with undesired payloads.
By providing antibodies that exhibit minimal cross-reactivity, GeneMedi empowers researchers with precise tools for designing and optimizing ADCs for targeted cancer therapy, ultimately advancing the forefront of biomedical research and clinical applications.
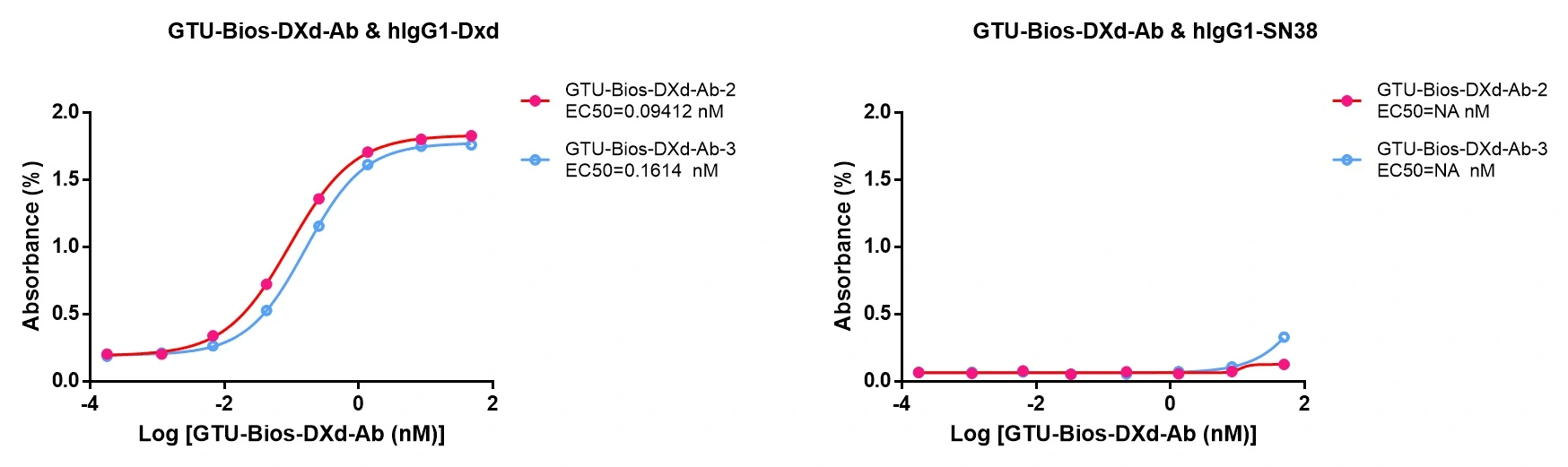
Figure 5. GeneMedi's GTU-Bios-DXd-Ab: Specific Binding with DXd, Not Binding with SN38.
GeneMedi's GTU-Bios-DXd-Ab exhibits specific binding to DXd-based ADCs, demonstrating its effectiveness. Moreover, it does not bind to ADCs conjugated with SN38, underscoring GeneMedi's commitment to producing highly selective antibodies.
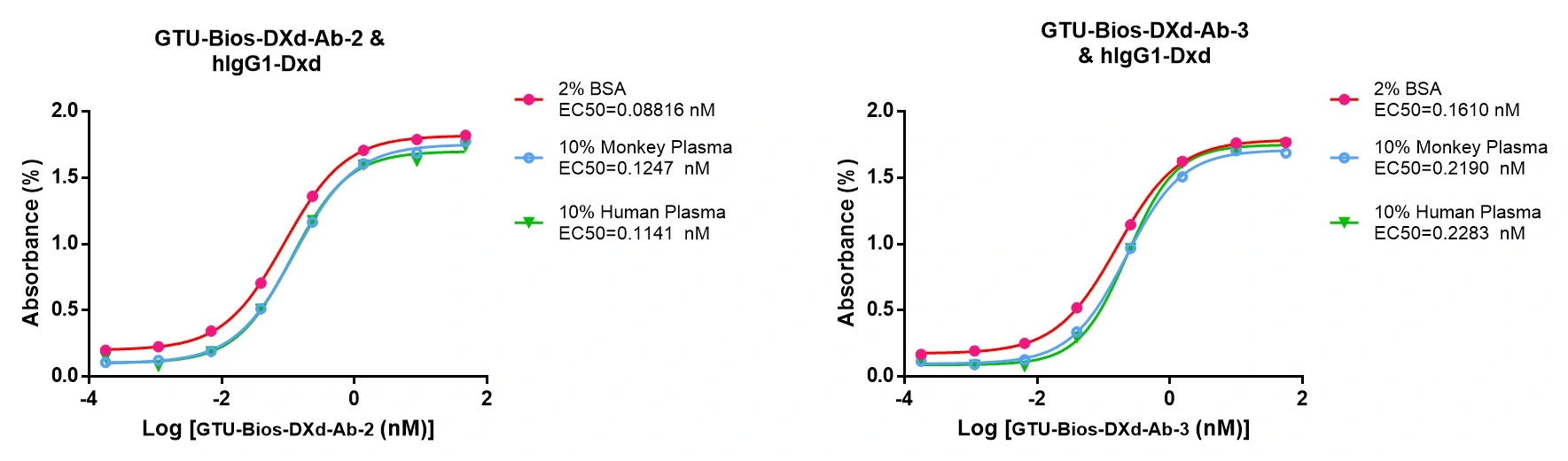
Figure 6. GTU-Bios-DXd-Ab has minimal impact on pharmacokinetic (PK) studies involving humans and monkeys.
GTU-Bios-DXd-Ab demonstrates minimal influence in PK studies with humans and monkeys, rendering it appropriate for application in these experiments.
GeneMedi’s Anti-Exatecan Antibodies Exhibit High Specificity
GeneMedi's GTU-Bios-Exatecan-Ab demonstrates specific binding affinity for Exatecan ADCs, highlighting its functional efficacy. Furthermore, its lack of binding to SN38-conjugated ADCs emphasizes GeneMedi's dedication to developing highly selective antibody solutions.
Figure 1. GeneMedi's GTU-Bios-Exatecan-Ab: Specific Binding with Exatecan, Not Binding with SN38.
A. Exatecan ADC binding activity assay results show that the EC50 of GTU-Bios-Exatecan-Ab-2 is 0.06271 nM, while the EC50 of GTU-Bios-Exatecan-Ab-3 is 0.07254 nM, indicating strong binding affinity for Exatecan ADC.
B. Both GTU-Bios-Exatecan-Ab-2 and GTU-Bios-Exatecan-Ab-3 are not available (N.A.) in the SN38 ADC binding assay.

The Strategic Use of Anti-payload antibody in ADCs
ADC therapies engage an “anti-payload antibody” and the mechanism and rationale utilised for the formation and advancement of ADC are rather different. Traditionally, ADC comprises of an antibody which is attached to a cytotoxic drug (payload) through a chemical linker. The antibody moiety of an ADC binds to specific antigens on cancer cells, releasing the toxic moiety exclusively on these cells and causing the destruction of the cancer cell without affecting the rest of the organism.
An “anti-payload antibody” for its part is an antibody that is known to bind firmly to a particular payload molecule. This could serve multiple purposes:
-
Safety and Toxicity Management: Specific to the structure to the cell, these antibodies that bind to free payload molecules, that maybe released non-specifically in the body, may help to reduce off target toxicity thereby making ADC therapy safer.
-
Enhanced Precision: They could be used research to enhance knowledge regarding biodistribution and clearance of the payload along with the intrinsic inabilities toward devising of more efficient and targeted ADC’s.
-
Drug Monitoring: Specifically, measuring the existence of anti-payload antibodies helps in creating assays that can be effectively used to calibrate the dosage of the drug according to individual patient requirements.
Although this concept is still in the embryonic stage in the field, the attempts to create anti payload antibodies are potentially enhancing the ADC technology next step with the goal to enhance the effectiveness of the treatment for cancer while reducing its impact on health.






 Facebook
Facebook LinkedIn
LinkedIn Twitter
Twitter
