Adenovirus vector system, Adenovirus production and transduction
 Adenovirus Protocol Download
Adenovirus Protocol Download
Adenovirus vector -Introduction
Landscape, protocol and guidelines of adenovirus vector system (adeasy and admax), adenovirus production, adenovirus mediated gene transduction in vitro and in vivo are described below. The detail information is mentioned about adenoviral vector (adenovirus plasmids) cloning, adenovirus packaging, purification and the insight of adenovirus gene delivery and gene therapy)
Adenovirus vector -Index
- 1. What is Adenovirus vector?
- 2. Adenovirus Gene Therapy Review
- 3. Advantages and Drawbacks of Adenovirus Vector-mediated Gene Transfer
- 4. Adenovirus Genome Structure (ITR, E1, E2A……) and adenovirus assembly
- 5. Life Cycle of Adenovirus-From Infection To Regeneration
- 6. Recombinant Adenovirus vector System (Adenovirus packaging System -AdMax system)
- 7. Adenovirus Vector Transduction -Adenovirus vector Gene Delivery in vitro and in vivo
- 8. Adenovirus vector-Production Protocol,Guidelines And References
Adenovirus vector-Knowledge Base
What is Adenovirus vector?
Adenovirus (AdV) is a member of the family Adenoviridae, whose name derives from their initial isolation from human adenoids in 1953 [1]. It is a medium-sized (90-100nm) and non-enveloped virus with an icosahedral nucleocapsid containing a double-stranded DNA genome. With a broad range of vertebrate hosts, 100 serotypes have been isolated, and about 57 distinct adenoviral serotypes in humans, causing a wide range of diseases, from mild respiratory infections in young children to life-threatening multi-organ disease in people with a weakened immune system. Since the isolation of adenoviruses, they were recognized as an invaluable tool for investigating mammalian molecular biology [2-4]. Considering the pathogenicity, recombinant adenovirus (rAdV), a replication-defective adenoviral vector system, is widely used for gene delivery in most cell types.
Based on human adenovirus type 5 (Ad5), recombinant adenovirus is replication-incompetent (-E1/-E3) and can’t be integrated into host genome, guaranteeing the security for subsequent operations [5]. With large cargo capacity (~8kb), and they are easily manipulated with recombinant DNA techniques. What’s more, recombinant adenovirus can efficiently transduce dividing and non-dividing cells and can be produced with high titers. All of these distinguishing features make adenovirus a preferred vehicle for gene delivery and transgene expression in mammalian cells [6].
2. Adenovirus Gene Therapy Review
Adenovirus has been proved as an excellent gene therapy vector. To date, more than 535 clinical trials have been carried out using adenovirus vectors for gene delivery [7], and promising gene therapy outcomes from recombinant adenovirus have been achieved from clinical trials for a great number of diseases (Table 1), especially for cancer treatment. Gene delivery by adenovirus is to “correct” and rebuild broken down system or infected tissues in vivo, and this kind of gene therapy has been used as an alternative in clinical trials today and proved to be effective in treatment of several cancers, including Prostate Cancer [8], Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL) [9], Non-small Cell Lung Cancer (NSCLC) [10], Melanoma [11], Renal cell carcinoma [12]. Among them, multimodal oncolytic adenoviruses have been used as biological therapeutics to promote tumor elimination in prostate cancer patients; adenovirus encoding chimeric CD154 (Ad-ISF35) can enhance the ability of CSL cells to function as antigen-presenting cells and increase their sensitivity to clearance based on immune-effector mechanisms; a large number of adenovirus-encoded transgenes (IL-1, GM-CSF, CD40L, IL-12, mda-7/IL24, IFN-gamma) vaccination could activate the immune system and induce cell death in an immunogenic fashion.
| Indication | Gene | Route of delivery | Phase | Sponsor |
| Hormone Refractory Prostate Cancer | PSA VACCINE | Intratumoral | II | David M Lubaroff |
| Prostate Cancer | hIL-12 | Intratumoral | Ⅲ | Baylor College of Medicine |
| Localized Prostate Cancer | REIC/Dkk3 | Intratumoral | I–II | Momotaro-Gene Inc. |
| Castration-resistant Prostate Cancer | DCVac/PCa | subcutaneously | I–II | Sotio a.s. |
| Recurrent Prostate Cancer | hIL-12 | Intratumoral | I | Simon Hall |
| PSA VACCINE | Intratumoral | Ⅲ | David M Lubaroff | |
| Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL) | ISF35 | Intranodal | I | University of California, San Diego |
| ISF35 | Intranodal | II | Januario Castro, M.D. | |
| CD19CAR | Intravenous | I | Baylor College of Medicine | |
| Non-small Cell Lung Cancer | yCD/mutTKSR39rep-ADP | intratumoral | I | Benjamin Movsas, M.D. |
| CCL21 vaccine | intratumoral | I | VA Office of Research and Development | |
| MAGEA3 | intratumoral | I–II | Turnstone Biologics, Inc. | |
| CCL21 vaccine | intratumoral | I | Jonsson Comprehensive Cancer Center | |
| Melanoma | CCL21 vaccine | intradermal | I | H. Lee Moffitt Cancer Center and Research Institute |
| RSV-TK | intratumoral | I | National Human Genome Research Institute (NHGRI) | |
| Renal cell carcinoma | DC vaccine | subcutaneously | I–II | Affiliated Hospital to Academy of Military Medical Sciences |
3. Advantages and Drawbacks of Adenovirus Vector-mediated Gene Transfer
a) Advantages of Adenovirus-mediated gene delivery
Adenovirus has been developed into a preferred candidate for creating viral vectors for gene therapy due to various advantages.
1) Well tolerated, with post-infection viability of the host cells being almost 100%.
2) Great packaging capacity (up to 8kb).
3) Broad range of infectivity. Adenovirus can infect both dividing and quiescent cells, allowing gene delivery to a highly diverse range of cell types.
4) It can be produced at high titer (10^10 VP/mL, which can be concentrated up to 10^13 VP/mL).
5) High infection efficiency. Almost 100% gene delivery in most cell types, completely surpassing other viral vector tools and liposome transfection.
6) Without integration into the host chromosome. Adenovirus remains epichromosomal in cells and does not inactivate genes or activate oncogenes.
b) Drawbacks of Adenovirus-mediated gene transfer
Although adenovirus benefits a great deal of disease therapies, it does present some drawbacks.
1) Adenovirus-mediated gene delivery may not sustain for long time, just transient expression.
2) Generation of neutralizing antibodies against adenovirus in the Non-Human Primates (NHP) and human, may attenuate the cure effect of adenovirus-mediated gene therapy [13].
3) Adenovirus vector infection can activate a wide variety of immune responses both humoral and cellular, which may increase the risk factor to use adenovirus as vectors in that high dose will result in acute toxicity and autoimmunity [14].
To date, the best solution for these drawbacks is to turn to Adeno associated virus (AAV) vectors, which can mediate long-term and stable expression and induce mild immune response, safer than adenovirus vectors.
| Comparison | Retrovirus | Lentivirus | Adenovirus | AAV |
| Genome | ss RNA | ss RNA | ds DNA | ss DNA |
| Integration | Yes | Yes | No | No |
| Packaging Capacity | 3kb | 4kb | 5.5kb | 2kb |
| Time to peak expression | 72h | 72h | 3h-72h | cell: 7 days; animals: 2 weeks |
| Sustainable time | about 3 weeks | stable expression | transient expression | > 6 months |
| Cell Type | most dividing/non-dividing Cells | most dividing/non-dividing Cells | most dividing/non-dividing Cells | most dividing/non-dividing Cells |
| Titer | 10^7 TU/ml | 10^8 TU/ml | 10^11 PFU/ml | 10^12 vg/ml |
| Animal experiment | suitable | low efficiency | lowest efficiency | most suitable |
| Immune Response | high | medium | medium | mild |
4. Adenovirus Genome Structure (ITR, E1, E2A……) and adenovirus assembly
Adenovirus is a non-enveloped, 90-100 nm diameter virus presenting icosahedral symmetry (Figure1A). Human adenovirus, containing a linear, double-stranded DNA genome, which is approximately 36kb, is wrapped in a histone-like protein and has two inverted terminal repeats (ITRs) of 50-200 bp, which act as origins of replication, accompanied by a complex series of splicing. Adenovirus transcription is a two-phase event, early and late, occurring before and after viral DNA replication, respectively (Figure1B). The early transcribed regions are E1A, E1B, E2A, E2B, E3, and E4, which are successively transcribed early in the viral reproductive cycle. The proteins coded for by genes within these transcription units are mostly involved in regulation of viral transcription, in replication of viral DNA, and suppression of the host response to infection. The late transcribed genes are L1-L5, which are transcribed later in the viral reproductive cycle, and encode mostly for proteins that make up components of the viral capsid or are involved in assembly of the capsid. The genes in the adenovirus and their functions are given in the following table 3.
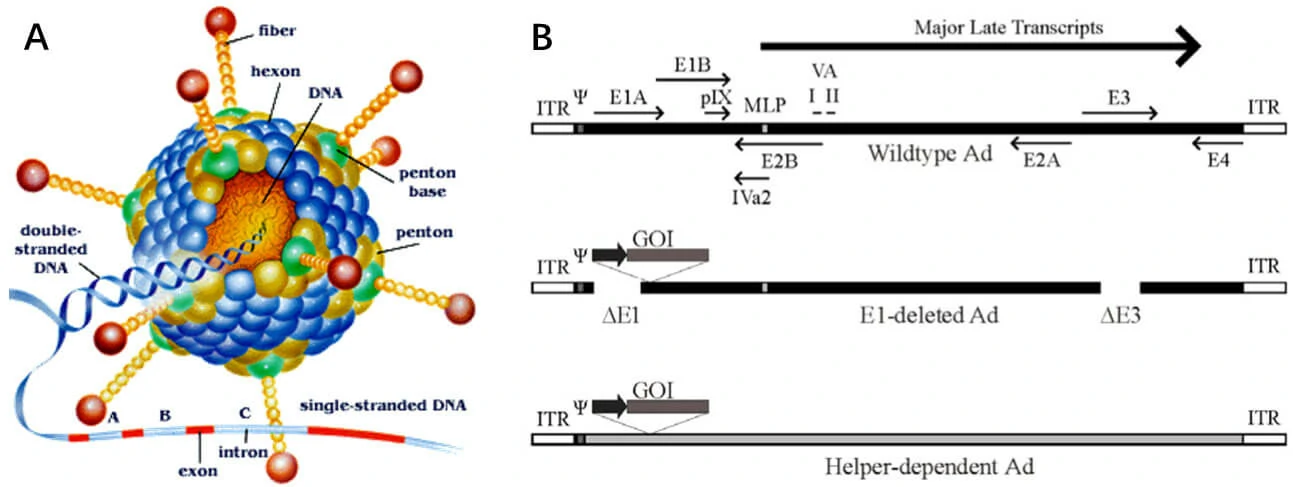
Figure1. Schematic of the adenovirus genome and adenovirus-based vectors. (A) Capsid crystal structure. (B) Adenovirus gene map. Top panel: A simplified map of the adenovirus serotype 5 genome showing the early genes (E1–E4) and the region from which the major late transcript is produced. Middle panel: General structure of an early region 1 (E1)-deleted Ad vector. Bottom panel: General structure of a helper-dependent Ad vector [15].
| Adenovirus protein | Functions |
| Capsid proteins II, III, IIIa, IV, VI, VIII, and IX | Structural proteins |
| Core proteins V, VII, X | Structural proteins |
| Terminal protein TP | Structural proteins |
| IVa2, 52K, L1, and 100K | Encapsidation proteins, assembly of viral capsids |
| L3 protease | Cleave viral precursor proteins pTP, pVI, pVII, pVIII, and IIIa to produce the mature viral proteins |
| E1A | Activate transcription of a number of viral genes as well as genes of the host cell |
| E1B 55K | Bind to and inactivate the transcriptional regulator p53, thus blocking transcription of genes normally activated by p53 and contributing to the suppression of apoptosis |
| E2A and E2B | Involve in replication of viral DNA |
| E3 RIDα and E3 RIDβ | Membrane proteins, perform a variety of molecular functions that contribute to inhibiting apoptosis |
| CR1β | Membrane glycoprotein, modulate the host immune response |
| E3 gp19K | Membrane glycoprotein, inhibit the insertion of class I MHC proteins in the host-cell membrane, thereby preventing T-cell lymphocytes from recognizing that the host cell has been infected by a virus |
| E3 14.7K | Protect the virus from host antiviral responses |
| E4 transcription unit | Involve in regulating transcription of viral DNA |
5. Life Cycle of Adenovirus-From Infection To Regeneration
For most serotypes, adenovirus infection is mediated by the high-affinity binding of the fiber-knob region to a receptor of target cell, named as the coxsackie-Ad receptor (CAR) [17]. Upon attachment, interaction between the penton-base Arg-Gly-Asp (RGD) and cellular αv integrins, which can stimulate actin polymerization, leads to internalization of the virus into the endosome. Then the endosome acidifies, resulting in disassociation of capsid proteins and transportation of viral DNA into nucleus. Without integration into host genome, adenovirus genome remains in an episomal state, which guarantees the low risk of mutation (Figure 2). Life cycle of adenovirus is separated by DNA replication process into two distinct phases: the early and late, occurring before and after viral DNA replication, respectively. After the synthesis of viral genome and capsid, they are assembled into viral products, releasing out of cell, and the infected cell starts lysis [18].
To prevent infected cell lysis, recombinant replication deficiency virus has been developed as a gene delivery tool to replace wild-type adenovirus (Figure 1B). Once packaged into a E1-complementing cell line, which provides the E1 products in trans, such as QBI 293A Cells, recombinant viral will be easily propagated.
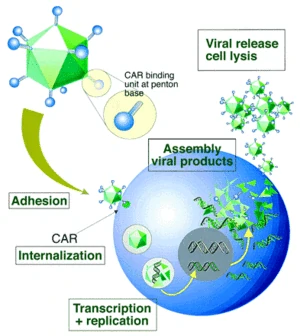
Figure 2. Infection process of adenovirus. CAR receptor-fiber-knob adenovirus interaction and internalization process [19].
6. Recombinant Adenovirus vector System (Adenovirus packaging System -AdMax system)
a) Introduction of recombinant adenovirus vector system
Since wild-type adenovirus is associated with a wide range of illnesses and enlists a variety of immune responses, so recombinant replication deficiency virus has been an attractive vector for gene therapy [20]. To date, there have been many different generations of adenovirus vectors, differing in the extent to which the genome from wild-type adenovirus is attenuated, among them, most studies involving adenovirus vectors utilize the simple E1-deleted, first-generation adenovirus vector. Due to the induction of strong innate and adaptive immune responses and limited the duration of transgene expression of first-generation adenovirus vector, researchers have further developed the second-generation adenovirus vectors by deleting the E2 or E4 coding sequences, resulting in some beneficial effects of reducing the expression of viral proteins and vector-directed immune responses. However, because of their limited evidence of enhanced efficacy over first generation vectors, the third-generation adenovirus vectors have been designed by deletion of all viral protein coding sequences, which can only be propagated in the presence of a second virus that provides all of the replication and packaging functions in trans, for which the third-generation adenovirus vectors are also known as fully-deleted, gutted, or helper-dependent Ad (hdAd) vectors [15].
Traditionally, recombinant adenovirus vectors used in gene delivery were prepared with a plasmid containing the transgene flanked by inverted terminal repeats (ITRs), co-transfected with packaging plasmid pAd-BHGlox(delta)E1, E3. Once packaged into a E1-complementing cell line, such as QBI 293A cells, recombinant viral will be easily propagated.
b) Recombinant adenovirus system
The current method of the recombinant adenovirus production is based on Adeasy and Ad.MAX™ system. It involves the co-transfection of 2 plasmids into QBI-293A cells as shown in Figure 3.
1. pAd-EF1-MCS-CMV-EGFP: an adenovirus ITR-containing plasmid carrying multiple clone sites, which can be cloned into a transgene;
2. pAd-BHGlox(delta)E1, E3: a packaging plasmid.
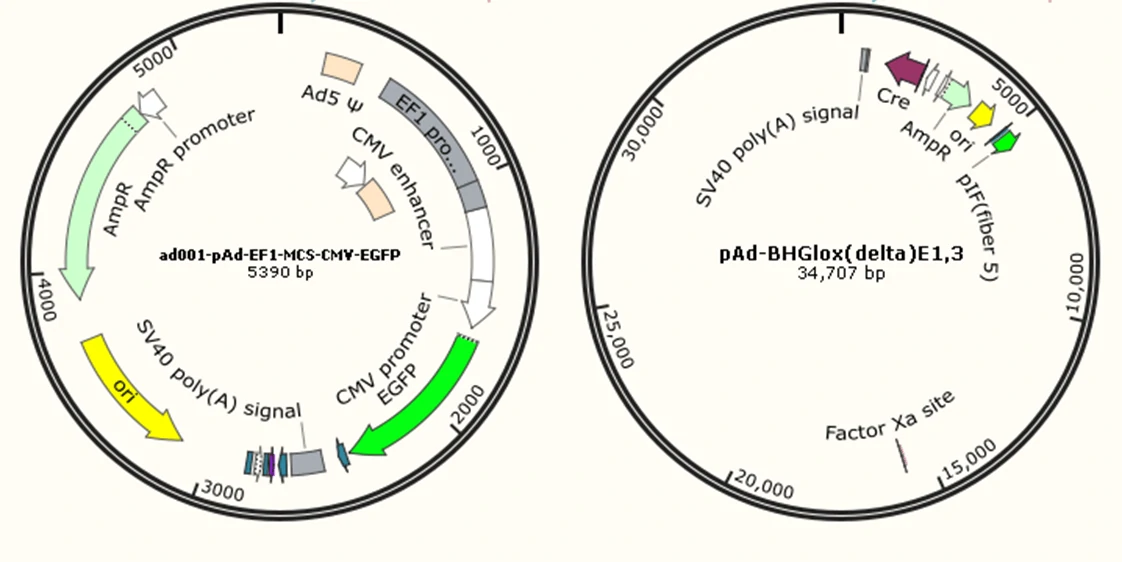
Figure3. The two plasmids co-transfection system of recombinant adenovirus.
7. Adenovirus Vector Transduction -Adenovirus vector Gene Delivery in vitro and in vivo
Adenovirus Infection Protocol in vitro and in vivo
a. Adenovirus Infection Protocol--Genemedi
For the reason that MOI varies in different cell lines, preliminary experiment is necessary to ensure a proper MOI of target cells before conducting formal experiments.
Note: MOI, multiplicity of infection, is the number of viral particles to infect one cell. An optimization test of MOI is strongly recommended as the real MOI to certain cells may be affected by methods of handling viruses in different labs.
1. Cell PreparationPlate robust target cells into 24-well plates at a density of 1 x 10^5/ml.
Note: The number of planted cells depends on the growth rate of a specific cell line. It should reach 50% to 70% confluence on the following day.
2. Transduction
Prepare the virus in 10-fold dilution gradient, and ensure the MOI is within a range of 3 to 1000.
For adherent cells:
rAdVs containing target gene and same amount of control viruses should be added separately into two groups of cells and mixed well. The amounts of viruses to be used are based on size of container described in the following table. For MOI test in most cell types, a gradient of 3, 10, 30, 100, 300 to1000 at three replicates would be sufficient enough. Refresh medium in 4 to 8 hours. Protein of interests can be detected within 48-72 hours with fluorescence microscopy or WB, etc.
| Size of Container | Surface Area (cm2) | Volume of Medium | Volume of Viruses |
| 96-well | 0.3 | 100 µl | 0.1-0.5 µl |
| 24-well | 2 | 500 µl | 1-3 µl |
| 12-well | 4 | 1 ml | 2-5 µl |
| 6-well | 10 | 2 ml | 5-20 µl |
For example: If the tier of rAdV is 5 x 10^11 PFU/ml, dilute to 5 x 10^10 PFU/ml (10-fold) with complete growth medium of target cells. When there are 1 x 10^5 cells in one well, and the MOI is 1,000, required volume of diluted virus (5 x 10^10 PFU/ml) should be (cell number) x (MOI) ÷ (PFU/ml of rAdV) = 1 x 10^5 x 1000/5 x 10^10 (ml) = 2 µl. Thus, 2 ul of diluted virus should be added into this well.
For suspension cells:
Spin infection is a sufficient way to transduce suspension or semi-suspension cells. In brief, seal the cell culture plate by parafilm after adding viruses, spin in a low-speed swinging-bucket centrifuge at 200 g for 1 hour at 37℃, and culture cells at 37℃ overnight. Medium should be refreshed the next day.
If the condition is not allowed for spin infection, a centrifuge tube can be used instead by transferring cells into a tube and centrifuging at low-speed. Discard most of the supernatant after centrifugation, add viruses, and incubate at room temperature for 15-30 min. Then transfer the cell-virus mixture into a proper container, and culture at 37℃ overnight. Medium should be replaced the next day.
3. Determine Transduction Efficiency
48 to 72 hours post-transduction, fluorescent proteins can be observed when applicable, and the alteration of target gene can be analyzed at mRNA-level by qPCR or at protein-level by WB.
b. Animal experiment
Caution: Purification of rAdV is required for animal injection.
Take intravenous (i.v.) injection of mouse as an example:
Each mouse is injected with 5×109 - 1×1010 purified viral particles. If the titer is 1×1011 PFU/ml, 50-100 µl of purified rAdV will be required for each mouse. The amount of virus used for different experiments should be optimized accordingly.
Note: the volume of i.v. injection is usually 100 µl and can’t exceed 200 µl. If too much liquid is injected, the mouse is prone to congestive heart failure. Please consult our technical support for more information on other animal experiments using rAdV.
Detailed information of Adenovirus protocol can be seen in Adenovirus Protocol.
8. Adenovirus Vector Transduction -Adenovirus vector Gene Delivery in vitro and in vivo
Adenovirus Production Protocol--Genemedi
 Adenovirus Protocol Download
Adenovirus Protocol Download
1. Adenovirus plasmid construction
The gene of interest is cloned into one of the ITR/MCS-containing adenovirus vectors to generate pAd -GOI. The purity and RNA contaminants of viral plasmid should be taken into consideration.
2. Adenovirus packaging
The recombinant adenovirus viral plasmid pAd-GOI is co-transfected into the 293A with pAd-BHGlox(delta)E1,3, which together supply all the trans-acting factors required for adenovirus replication and packaging in the293A cells.
3. Plaque formation and cell collection
Pick an isolated viral plaque together with surrounding agarose, and transfer into 1 ml fresh medium and incubate overnight. In general, three to six plaques should be picked to compare their virus titer, then the one with the highest titer will be proceeded into subsequent experiments.
4. Virus amplification
On the next day, add virus-containing supernatant into fresh, pre-seeded 293A cells to amplify virus. Collect cells and supernatant when observing formation of plaques, and proceed into a freeze-thaw cycle 3 times before collecting all viruses. The collected virus is recognized as passage 1 (P1 virus). Then, infect fresh 293A cells with P1 virus. Perform infection-collection cycle three times till P4 virus is obtained, and expand virus production into large-scale through P4 virus infection. When formation of plaques is observed, viruses are collected for purification and concentration.
5. Adenovirus purification
The purification process of rAdV is composed of three steps: PEG8000 condensation, CsCl density gradient centrifugation and dialysis. Add 50 ml PEG8000 solution (20% PEG8000 in ultra-pure water with 2.5 M NaCl) per 100ml virus supernatant, placing on ice for 1 hour to pull-down viruses. Centrifuge the mixture, discard the supernatant and resuspend virus pellet in 10 ml CsCl solution at density of 1.10g/ml (the virus-containing CsCl solution should be pink). Then, carry out CsCl density gradient centrifugation with Beckman SW28 rotor at 26,000 rpm, 4℃ for 2 hours. Collect virus band between 1.30g/ml and 1.40g/ml layers with a syringe, transfer into dialysis bag. Put the dialysis bag containing virus in dialysis buffer, and stir at 4℃ overnight. Replace with fresh buffer once during dialysis. Collect virus from the bag, adjust volume to 500 µl with PBS, and determine the titer. Purified rAdV should be kept at 4℃ for no more than a week or at -80℃ for long time storage.
6. Adenovirus titer detection
Viral titer is determined by plaque assay of rAdV. Plate 293A cells in 60-mm dishes 24 hours ahead. When cell confluence reaches approx. 100%, add diluted virus at different concentrations and incubate at 37℃ 4 to 8 hours after infection, then cover cells with 8 ml low-melting-point agarose solution (10% FBS, 1.25% agarose). Calculate the titer of rAdV by counting number of plaques in 9-11 days of culturing.
7. Quality control of adenovirus
After adenovirus titer detection, the infection activity needs to be evaluated before animal experiment by infecting cells such as 293T, CHO to test the gene expression.
Adenovirus - Guidelines
1.
Rowe WP, RJ Huebner, LK Gilmore, RH Parrott and TG Ward. (1953). Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med 84:570-3.
2.
Kumon H, Y Ariyoshi, K Sasaki, T Sadahira, M Araki, S Ebara, H Yanai, M Watanabe and Y Nasu. (2016). Adenovirus vector carrying REIC/DKK-3 gene: neoadjuvant intraprostatic injection for high-risk localized prostate cancer undergoing radical prostatectomy. Cancer Gene Ther 23:400-409.
3.
Guan YS, Y Liu, Q Zou, Q He, Z La, L Yang and Y Hu. (2009). Adenovirus-mediated wild-type p53 gene transfer in combination with bronchial arterial infusion for treatment of advanced non-small-cell lung cancer, one year follow-up. J Zhejiang Univ Sci B 10:331-40.
4.
Butterfield LH, B Comin-Anduix, L Vujanovic, Y Lee, VB Dissette, JQ Yang, HT Vu, E Seja, DK Oseguera, DM Potter, JA Glaspy, JS Economou and A Ribas. (2008). Adenovirus MART-1-engineered autologous dendritic cell vaccine for metastatic melanoma. J Immunother 31:294-309.
5.
Appaiahgari MB and S Vrati. (2015). Adenoviruses as gene/vaccine delivery vectors: promises and pitfalls. Expert Opin Biol Ther 15:337-51.
6.
Walsh MP, J Seto, EB Liu, S Dehghan, NR Hudson, AN Lukashev, O Ivanova, J Chodosh, DW Dyer, MS Jones and D Seto. (2011). Computational analysis of two species C human adenoviruses provides evidence of a novel virus. J Clin Microbiol 49:3482-90.
7.
Vectors used in gene therapy clinical trials. The Journal of Gene Medicine Online Library. [Online] Updated Nov 2017.
8.
Sweeney K and G Hallden. (2016). Oncolytic adenovirus-mediated therapy for prostate cancer. Oncolytic Virother 5:45-57.
9.
Castro JE, J Melo-Cardenas, M Urquiza, JS Barajas-Gamboa, RS Pakbaz and TJ Kipps. (2012). Gene immunotherapy of chronic lymphocytic leukemia: a phase I study of intranodally injected adenovirus expressing a chimeric CD154 molecule. Cancer Res 72:2937-48.
10.
Predina JD, J Keating, O Venegas, S Nims and S Singhal. (2016). Neoadjuvant intratumoral immuno-gene therapy for non-small cell lung cancer. Discov Med 21:275-81.
11.
Schiza A, J Wenthe, S Mangsbo, E Eriksson, A Nilsson, TH Totterman, A Loskog and G Ullenhag. (2017). Adenovirus-mediated CD40L gene transfer increases Teffector/Tregulatory cell ratio and upregulates death receptors in metastatic melanoma patients. J Transl Med 15:79.
12.
Garcia-Carbonero R, R Salazar, I Duran, I Osman-Garcia, L Paz-Ares, JM Bozada, V Boni, C Blanc, L Seymour, J Beadle, S Alvis, B Champion, E Calvo and K Fisher. (2017). Phase 1 study of intravenous administration of the chimeric adenovirus enadenotucirev in patients undergoing primary tumor resection. J Immunother Cancer 5:71.
13.
Eichholz K, T Bru, TT Tran, P Fernandes, H Welles, FJ Mennechet, N Manel, P Alves, M Perreau and EJ Kremer. (2016). Immune-Complexed Adenovirus Induce AIM2-Mediated Pyroptosis in Human Dendritic Cells. PLoS Pathog 12:e1005871.
14.
Gao GP, Y Yang and JM Wilson. (1996). Biology of adenovirus vectors with E1 and E4 deletions for liver-directed gene therapy. J Virol 70:8934-43.
15.
Wong CM, ER McFall, JK Burns and RJ Parks. (2013). The role of chromatin in adenoviral vector function. Viruses 5:1500-15.
16.
Russell WC. (2009). Adenoviruses: update on structure and function. J Gen Virol 90:1-20.
17.
Ranki T and A Hemminki. (2010). Serotype chimeric human adenoviruses for cancer gene therapy. Viruses 2:2196-212.
18.
Ison MG and RT Hayden. (2016). Adenovirus. Microbiol Spectr 4.
19.
Vorburger SA and KK Hunt. (2002). Adenoviral gene therapy. Oncologist 7:46-59.
20.
Amalfitano A and RJ Parks. (2002). Separating fact from fiction: assessing the potential of modified adenovirus vectors for use in human gene therapy. Curr Gene Ther 2:111-33.







 Facebook
Facebook LinkedIn
LinkedIn Twitter
Twitter
