GM AAV packaging services
- Efficient and Productive/ Unmatched Quality and Viability/ Diverse AAV Vector Grades/ Rapid Turnaround, High Purity, Enhanced Activity, and Superior Safety
Quick Quote AAV GOI Production Guide
GVC AAV Production Service
AAVs are known to be safe and efficient viral vectors for gene therapy and gene editing tasks. Viral vectors are specifically suitable for in vivo gene transfer. With the development of three generations of AAV from AAV1.0, AAV2.0 to AAV3.0, there are more and more artificial serotypes with different tissue preferences, which enable specific targeting to the desired organs.
GVC (GeneMedi Vector Core) has several AAV vector grades that range from research to clinical to ensure that there are different levels of AAV vectors for the various uses of scientific and clinical research. The production of GVC AAV vectors is efficient, productive, of high quality, and completely reliable due to the implementation of proprietary state-of-the-art technology, flexible and smooth production workflows, and strict quality control.
GeneMedi GVC will be an ideal partner for your AAV production and AAV process development.
Types of AAV in GVC service
-
Multipleserotypes: AAV1, 2, 3b, 4, 5, 6, 7, 8, 9, PHP.eB, PHP.B, PHP.S, rh10, DJ, DJ/8, AAV2.7m8, AAV2-retro,AAV8-1m/2m/3m, AAV2(Y444F), AAV2, AAV6.2FF, AAV-i.e, AAV-BR1, AAV-2i8, AAV-SIG, AAV-VEC, AAV-Myo, Anc80, etc.
-
Single-stranded AAV (ssAAV) and self-complementary AAV (scAAV)
-
Pre-made AAV particle and Customized AAV production
Stability of GVC AAV Production
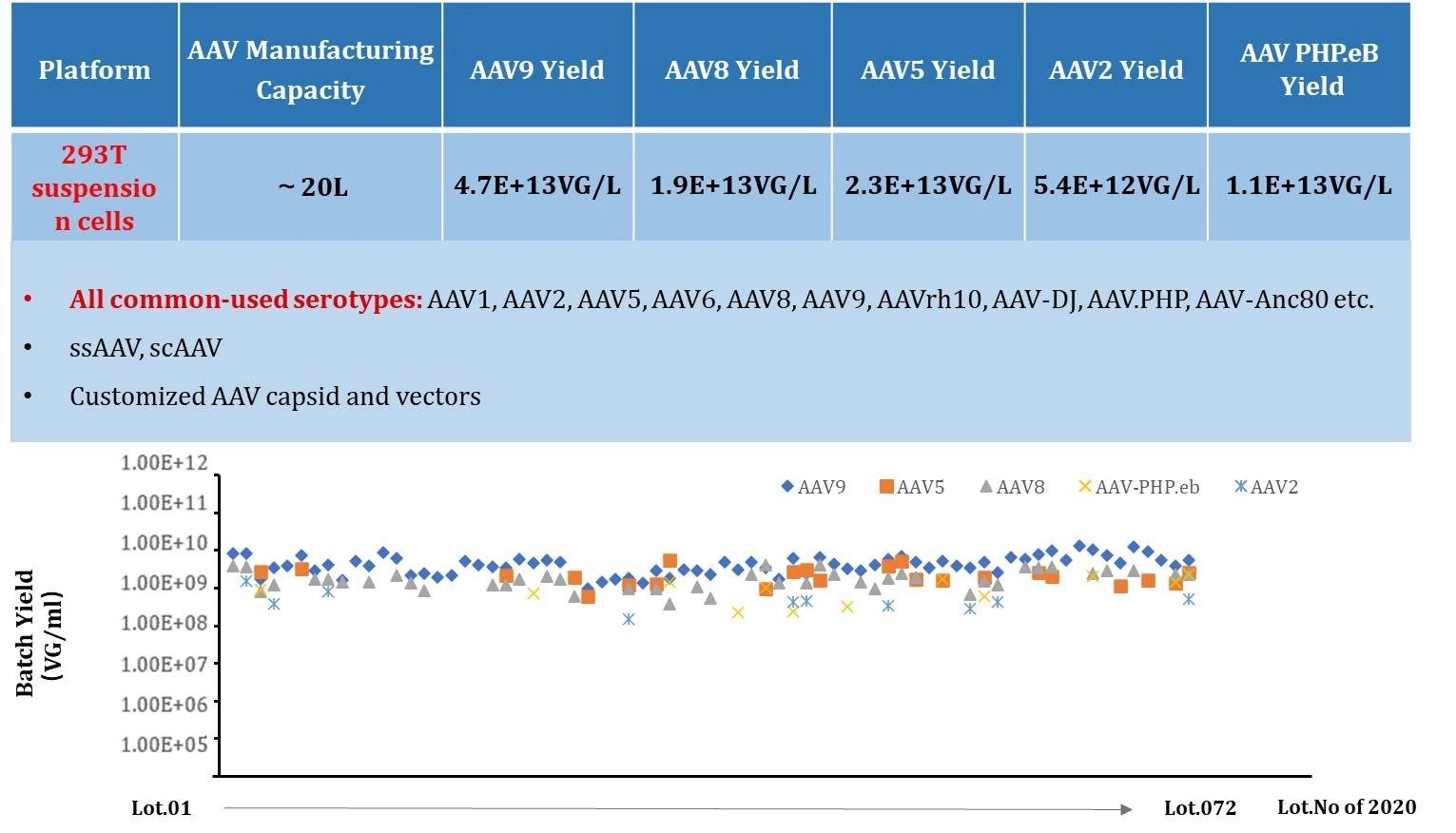
Figure 1: Consistent AAV Production Platform With High Yield.
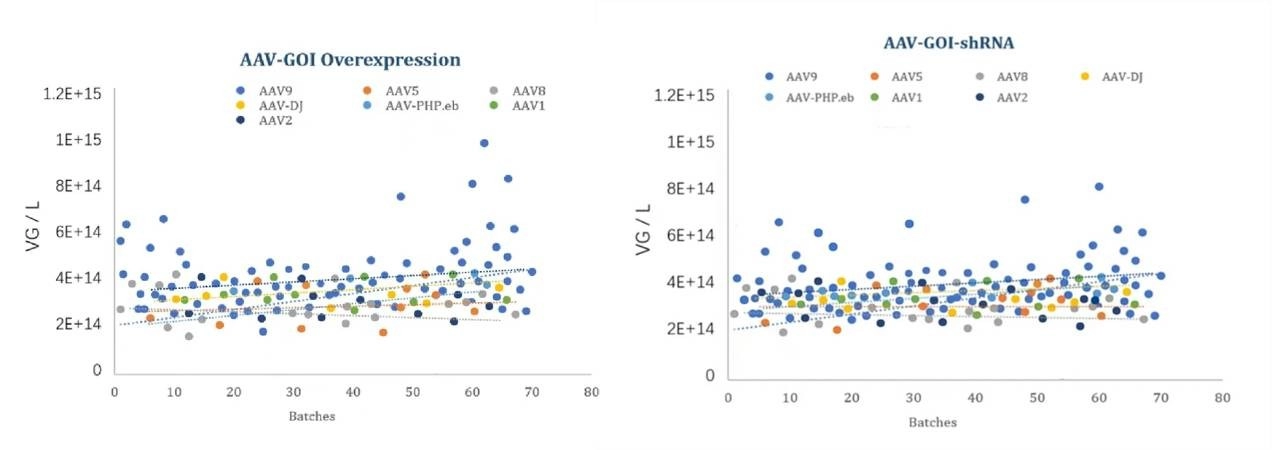
Figure 2: Manufacturing Batch Stability of Different AAV Serotypes
Upstream Process & Downstream Process
| Transient Triple Transfection | Research Grade-Pilot | Research Grade-Standard | GMP-like Grade/GMP Grade |
| Cell Type | Attached | Attached | Suspended |
| Cell Bank | —— | —— | TBD |
| Serum-Free | N | N | Y |
| Plasmid Bank | N | N | Y |
| Purification | Non-purification | CsCl density gradient | Clarification Tangential Flow Filtration Affinity chromatography Anion-Exchange (AEX) Chromatography |
| Scale Range | 1E+12 GC/~5E+13 GC | 5E+12 GC/~5E+14 GC | TBD |
Analycal Development & Quality Control (AD&QC)
- General inspection (physical inspection)
- Identify
- Content and potency
- Purity
- Security check
- Impurities
| QC project | Research Grade-Standard | GMP-Like Grade/GMP Grade | ||
| Method | Result | Method | Result | |
| Appearance, clarity | Light Inspection | Visual inspection "Clear solution, free of particulates" |
Appearance, clarity | Visual inspection "Clear solution, free of particulates" |
| Visible foreign matter | —— | —— | Visible foreign matter | Lamp inspection |
| Insoluble particles | —— | —— | Insoluble particles | Light blockage method |
| pH value | —— | —— | pH value | pH meter Product specific |
| Osmotic pressure | —— | —— | Osmotic pressure | Cryoscopic method Excipient specific |
| Loading volume | —— | —— | Loading volume | Volumetric Method-Meets USP/ChP Requirements |
| QC project | Research Grade-Standard | GMP-Like Grade/GMP Grade | ||
| Method | Result | Method | Result | |
| AAV genome sequence | —— | —— | Restriction enzyme map analysis | The result is subject to no mismatch |
| —— | —— | Polymerase chain reaction (PCR) | ||
| —— | —— | Reverse transcription-polymerase chain reaction (RT-PCR) | ||
| —— | —— | Nucleic acid sequence determination | ||
| Capsid protein identification | SDS-PAGE combined with Western blot to identify capsid protein VP1/2/3 | VP1: VP2: VP3=1: 1: 10 | SDS-PAGE combined with Western blot to identify capsid protein VP1/2/3 | VP1: VP2: VP3=1: 1: 10 |
| Identification of intact virus particles | —— | —— | Structural analysis, particle size distribution, refractive index analysis under electron microscope | Aggregates-TEM (negative stain)≤10% |
| QC project | Research Grade-Standard | GMP-Like Grade/GMP Grade | ||
| Method | Result | Method | Result | |
| Virus particle titer (V.P.) | —— | —— | ELISA | Report result/~1E14 VP/ml |
| Genomic Titer (V.G.) | QPCR | The titer detected by Q-PCR is ≥ the protocol amount | ddPCR | Reported result/~1-5E13 VG/mL |
| Total protein | —— | —— | BCA | Reported result |
| Protein expression (Cell) | —— | —— | ELISA/FIX | TBD |
| Infectious titer (I.P.) | —— | —— | TCID50 | Report result/1-5E11 TCID50/ml |
| Specific activity | —— | —— | Specific activity = genome titer/infectious titer | <100 |
| Potency - Genomic Expression | —— | —— | Detection of in vitro mRNA expression or protein expression of cells based on qPCR, ELISA, and Western Blot | Potency Content UV 0.8-1.2 mg/mL |
| Potency-activity | —— | —— | Bio-functional assay | In Vitro Potency- (between 1-15) vg/cell |
| QC project | Research Grade-Standard | GMP-Like Grade/GMP Grade | ||
| Method | Result | Method | Result | |
| Capsid protein purity test | SDS-PAGE electrophoresis combined with silver staining | SDS-PAGE ≥95%, VP1: VP2: VP3=1:1:10, no obvious contaminants were detected. | SDS-PAGE electrophoresis combined with silver staining | SDS-PAGE ≥95%, VP1: VP2: VP3=1:1:10, no obvious contaminants were detected. |
| Empty shell rate detection | —— | —— | ddPCR/ ELISA | Report result |
| —— | —— | Transmission electron microscope (TEM) | Report result | |
| —— | —— | Analytical ultracentrifugation (AUC) | Report result | |
| Genomic aggregation detection | —— | —— | Size exclusion high performance liquid chromatography (SEC-HPLC method) | Report result |
| QC project | Research Grade-Standard | GMP-Like Grade/GMP Grade | ||
| Method | Result | Method | Result | |
| Replication-Competent Adeno-associated virus, or rcAAV for short, is an important evaluation indicator of product safety. | —— | —— | DNA immunoblotting (Southern blot) and qPCR | qPCR Negative |
| Sterility test | Membrane filtration | No growth | Membrane filtration | No growth |
| Bacterial endotoxin test | Limulus reagent | TBD | Limulus reagent | LAL assay<10 EU/ml |
| Mycoplasma | Culture method and PCR method | TBD | Culture method and PCR method | Negative |
| Exogenous factors | —— | —— | Culture | Negative |
| Bioburden | —— | —— | Culture | Report result |
| Abnormal toxicity check | —— | —— | Animal experiment | Meets ChP Requirement |
| QC project | Research Grade-Standard | GMP-Like Grade/GMP Grade | ||
| Method | Result | Method | Result | |
| Help DNA plasmid residue | —— | —— | ddPCR/qPCR | ≤50ng/ml |
| Host cell DNA residue | —— | —— | ddPCR/qPCR | ≤50 ng/ml |
| Residual E1A/E1B DNA(copies/dose) | —— | —— | ddPCR/qPCR | ≤(5-10) x 10^4 copies/mL E1A; (5-10) x 10^4 copies/mLE1B |
| Host cell protein residue | —— | —— | ELISA | ≤0.5ug/10E^12/ml |
| Bovine serum albumin residue | —— | —— | ELISA | ≤50ng/ml |
| Residual Benzonase | —— | —— | ELISA | ≤10 ng/ml |
| Residues related to specific processes (such as iodixanol, transfection reagent, affinity ligand, Tween 20, detergent TritonX100, etc.) | —— | —— | HPLC | Report result |
Workflow of AAV Production

About GVC
GVC (GM Vector Core) is GeneMedi's unique platform for QbD Viral vectors Processes development and manufacturing. In GVC, our core expertise lies in the tailored production of viral vectors, including adeno-associated virus (AAV), lentivirus, adenovirus and VLPs.
Our state-of-the-art facilities are equipped for scalable manufacturing, ensuring high-quality viral vector production to meet both research and therapeutic needs. Our expert team specializes in process development, leveraging innovative technology and extensive industry knowledge to provide clients with tailored solutions that exceed expectations.
GVC will be the ideal partner for scientists and healthcare professionals seeking reliable and efficient viral vector production services.
AAVs Affinity Purification Efficiency
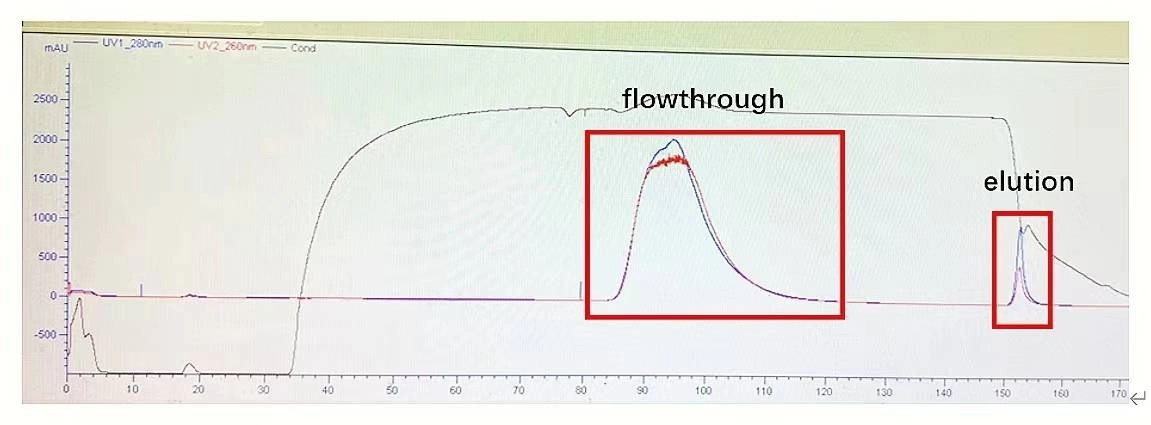
Figure 3. AAV5 affinity purification: The column efficiency of AAV affinity chromatography is very well.
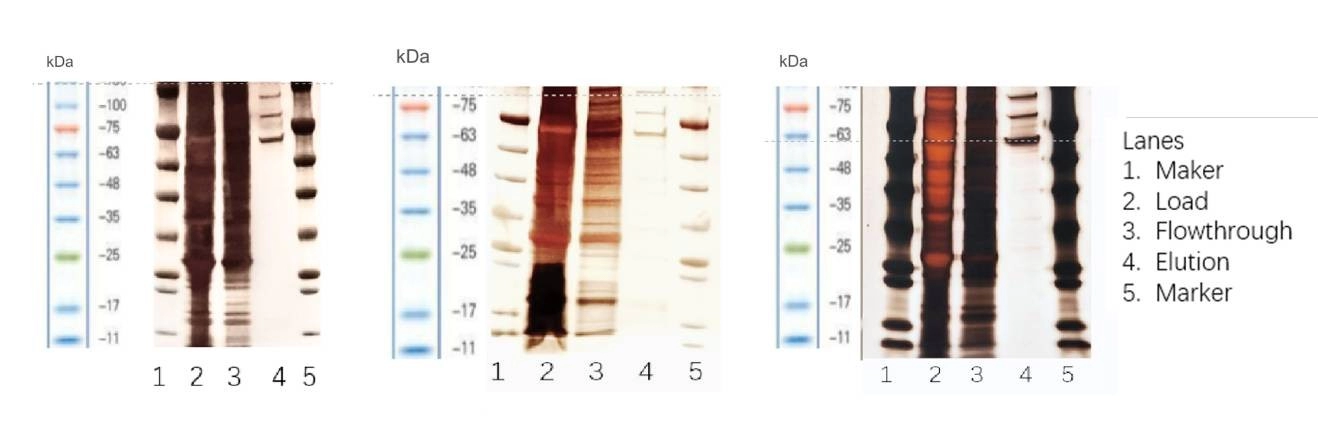
Figure 4. Silver staining: The purity after AAV5/ AAV8/ AAV2 affinity chromatography is high.
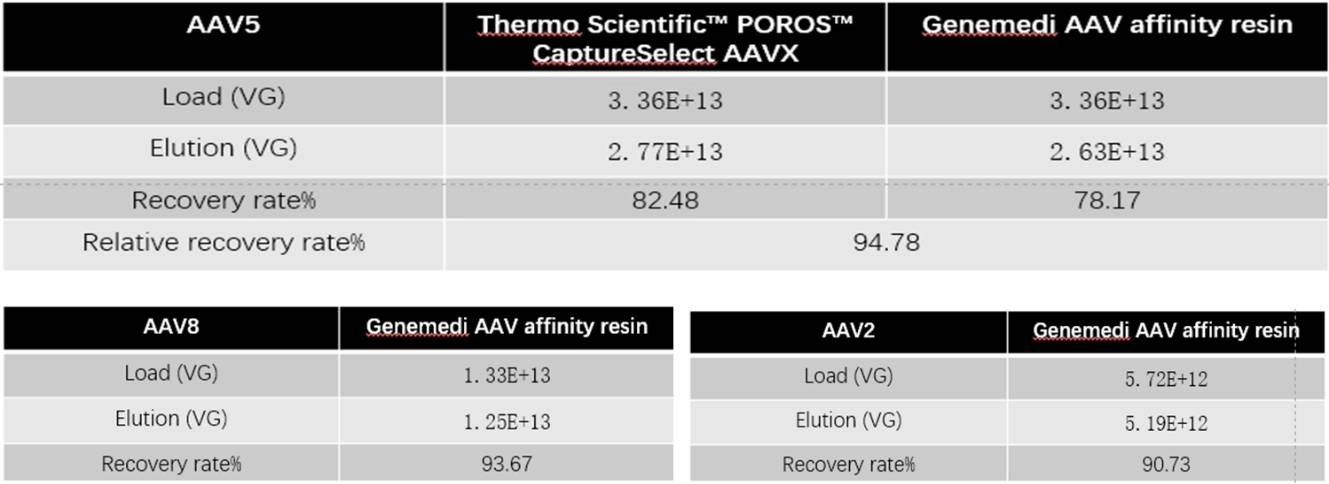
Table 1: ddPCR detect the titer compare with Thermo Scientific™ POROS™ CaptureSelect AAVX: The recovery rate of AAV affinity chromatography is comparable.
AAV Potency Enhancement
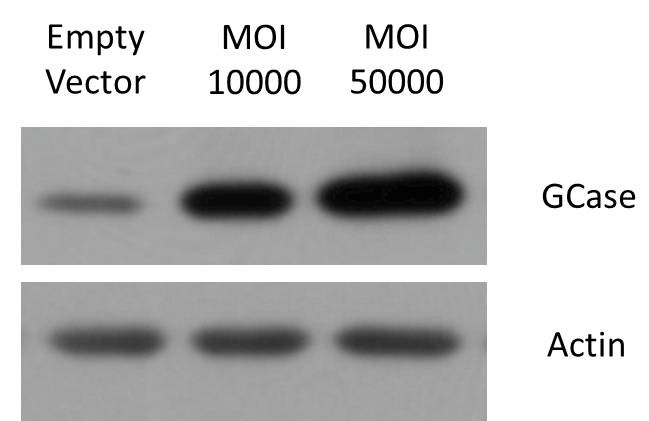
Figure 5. AAV Potency. WB results show that overexpressed viruses can significantly increase protein content
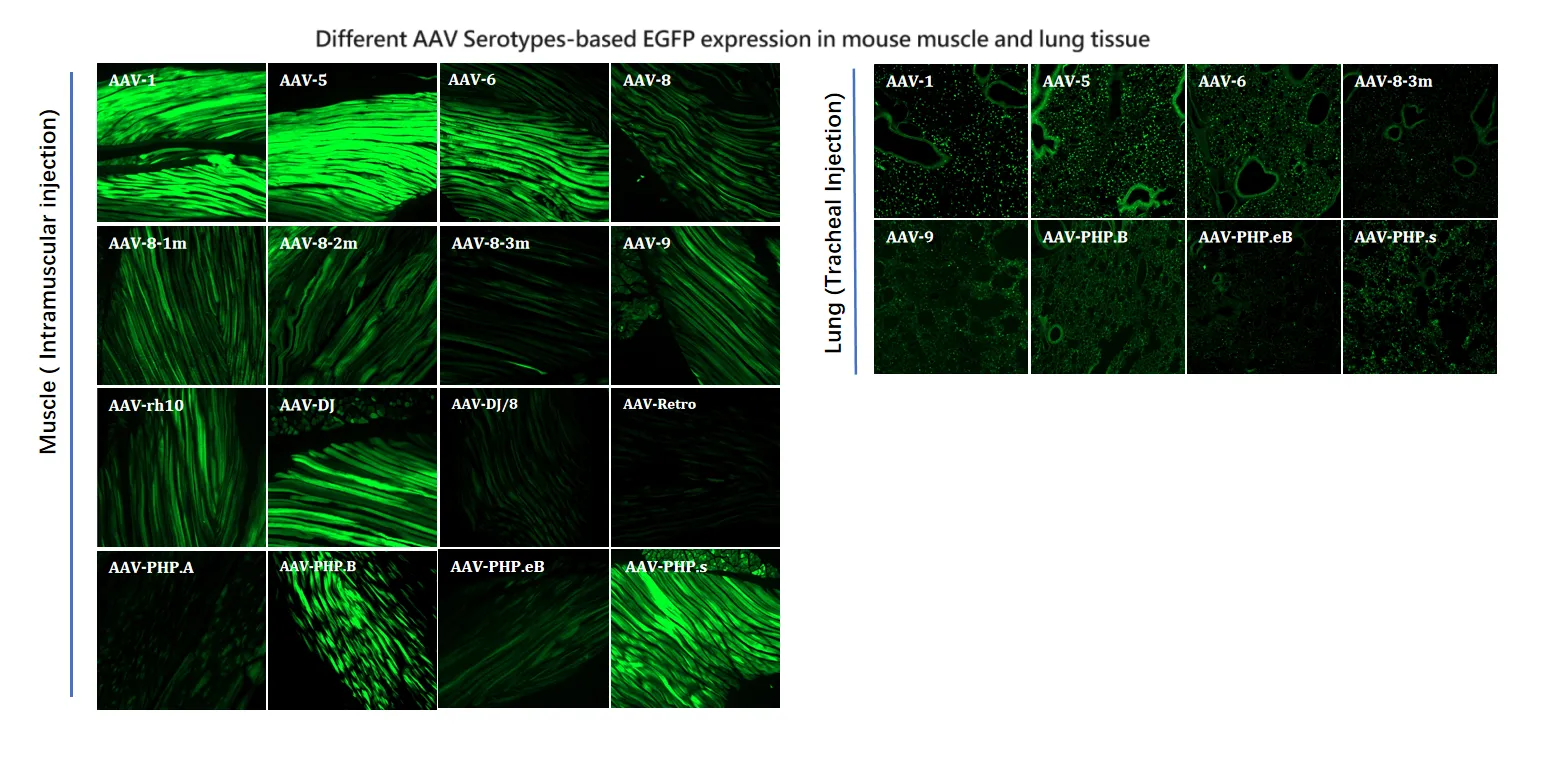
Figure 6. Different AAV Serotypes-based EGFP expression in mouse muscle and lung tissue

Figure 7. GM-AAV-ab-1 has the ability to bind to multiple serotypes
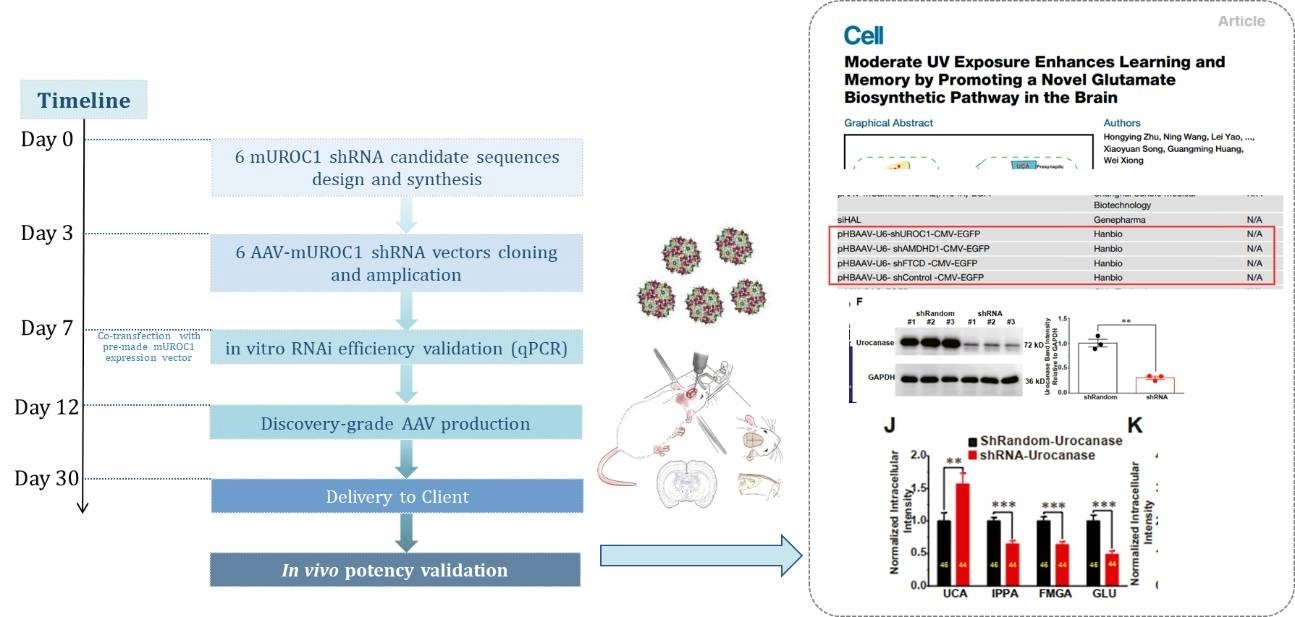
Case study 1: AAV-mediated UROC1-RNAi in mouse hippocampus
In a groundbreaking study, GeneMedi's AAV vectors were used to deliver UROC1-RNAi to the mouse hippocampus, demonstrating our products' pivotal role in advancing neuroscience research. This application highlights the precision and efficiency of our AAV vectors in targeting specific brain regions, opening new avenues for studying and treating neurological conditions.
Case study 2: Heart-specific AAV expression vector development
Our capabilities in AAV cassette optimization are showcased through the development of a heart-specific expression vector, emphasizing our tailored approach to gene therapy. This achievement underscores our expertise in promoter optimization, enabling precise control over gene expression in targeted tissues, crucial for therapeutic success and specificity.
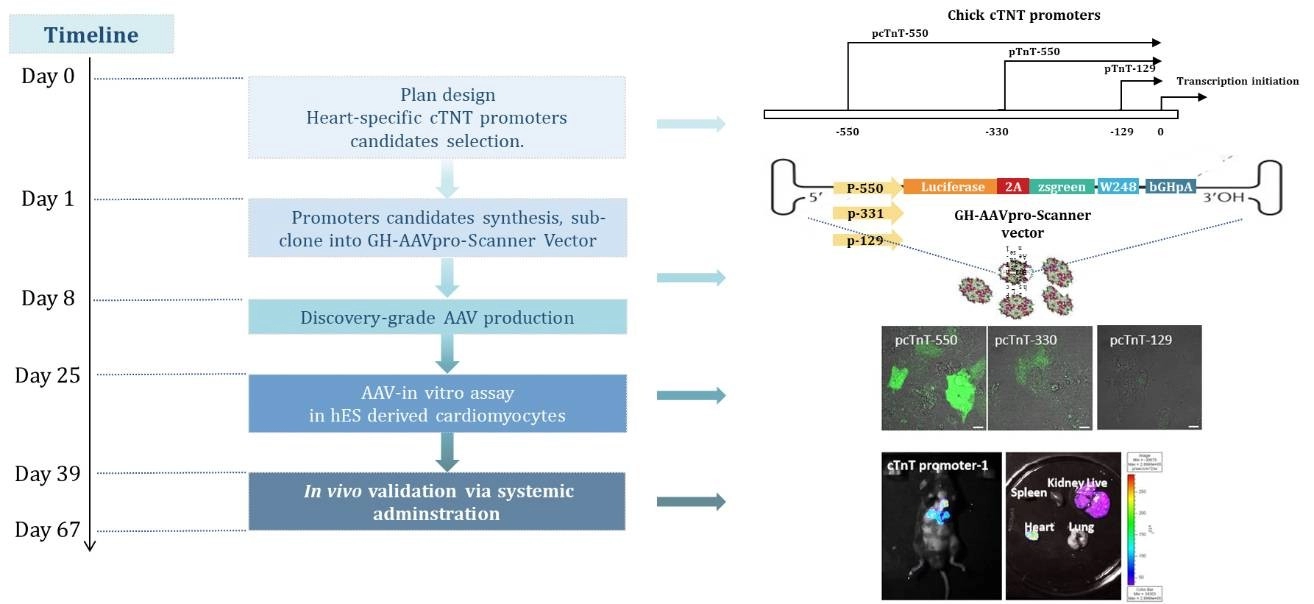
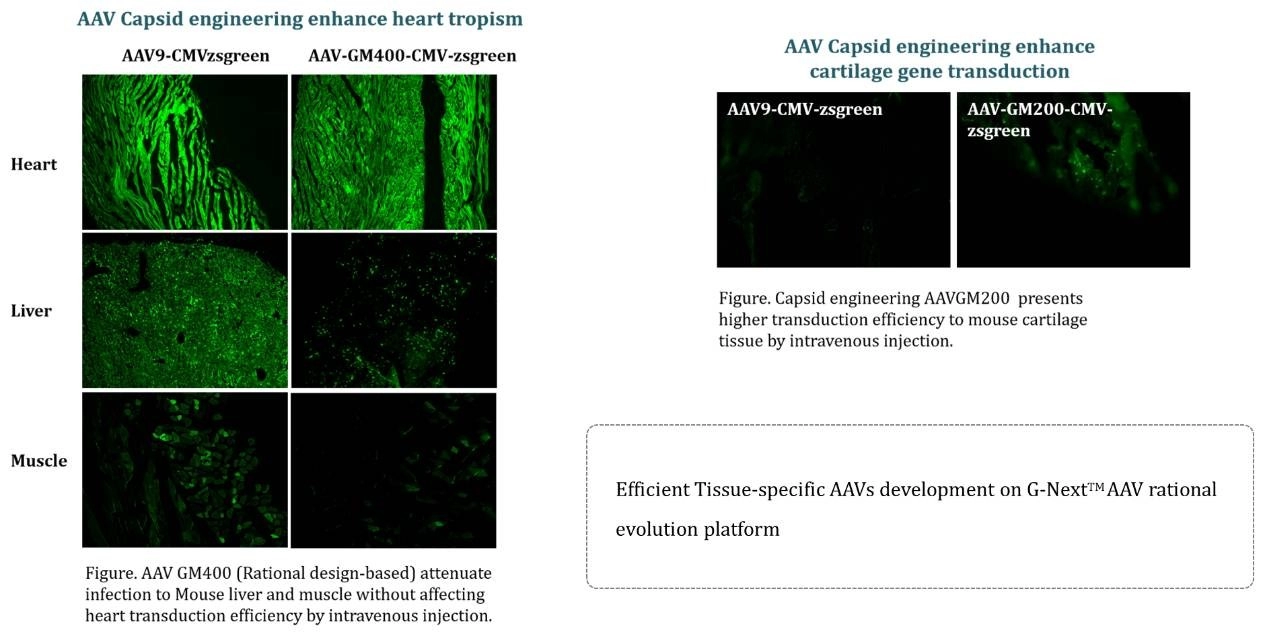
Case study 3: Better Tissue Specificity of Engineered AAV Vector From G-Next™
The engineered AAV vectors from GeneMedi's G-NextTM platform exhibit superior tissue specificity, demonstrating our innovative edge in vector design. This advancement enhances the precision of gene delivery to specific tissues, reducing off-target effects and increasing the therapeutic potential of gene therapies. Our commitment to innovation and quality underscores GeneMedi's role as a leader in the gene therapy field, driving forward the possibilities of personalized medicine.
Citation&Publication
|
Citation&Publication |




 Facebook
Facebook LinkedIn
LinkedIn Twitter
Twitter
